
분자, 원자, 원소, 입자(소립자), Molecules, atoms, elements, particles
- 분자 : 원자가 세트를 이뤄서 만들어진 물질 단위. ex) 물은 수소 원자 둘과 산소 원자 하나로 이루어진 분자
- 원자 : 원소를 통틀어 이루는 말. 화학적으로 쪼갤 수 있는 물질의 최소 단위. 원자는 전자, 중성자, 양성자 3개로 이루어짐.
- 원소 : 원자의 종류. 전자, 중성자, 양성자의 개수에 따라 달라짐. 원소를 나열한 것이 주기율표.
- 입자(소립자) : 물질의 최소 단위. 종류는 쿼크, 렙톤, 보손 3가지가 있음. 쿼크 6개, 렙톤 6개, 보손 5개로 총 17개가 있음. 이것들이 세트를 이뤄서 전자, 중성자, 양성자를 만듦.
원자와 원소
* '원자'와 '원소'를 혼동하기 쉬운데, 원자가 물질을 구성하는 기본 입자라고 한다면, 원소는 물질을 이루는 성분의 종류
- 원소(元素, 영어: element) : 화학적 방법으로 더 간단한 순물질로 분리할 수 없는 물질 (종류의 개념)
EX) 포도당(C6 H12 O6)은 물질의 기본적 구성요소인 탄소(C), 수소(H), 산소(O)의 3가지의 원소로 이루어져 있다. 메탄(CH4)은 탄소(C)와 수소(H)의 2개의 원소로 구성된다.

- 원자(原子, atom) : 일상적인 물질을 이루는 가장 작은 단위로 더 이상 쪼갤 수 없는 순물질의 구성 입자이다. (수의 개념)

■ 분자
분자(分子, 영어: molecule) : 물질을 구성하는 최소의 단위 혹은 화합물의 최소 단위를 일컫는다. 즉, 물질의 고유한 성질을 가지는 가장 작은 단위 입자이다.
- 비활성 기체는 단원자가 분자(그 자체로 원자이며 분자) : He, Ne, Ar
- 가장 크기가 작은 이원자 분자 : 수소(H2)
- 홑원소로 존재하는 분자(공유 결합) : 질소(N2), 산소(O2), 염소(Cl2)
- 서로 다른 원소끼리 결합한 분자 : 물 분자(H2O)
- 분자의 크기가 매우 큰 고분자 : 단백질, DNA
※ 분자라고 할 수 없는 것들이 있다.(규칙적 배열을 하는 화합물)
- 두 개 이상의 비금속 원자가 화학 결합에 의해 전기적으로 전하를 띄거나 중성을 띄는 원자 그룹 : NaCl은 이온 결합한 화합물 (1:1 비율로 균일하게 분포된 화합물)
- Fe, Na, Ca 금속화합물 등
위의 내용을 한눈에 볼 수 있도록 정리

순물질을 혼합물로 만들려면 혼합을 하면 된다. 반대로
혼합물을 순물질로 만들려면 분리(=물리적 변화)를 하면 된다.
홑원소 물질을 화합물로 만들려면 화학적 결합이 필요하다. 반대로
화합물을 홑원소 물질로 만들려면 분해(=화학적 변화)가 필요하다. (전기분해 등)
Atoms, molecules and ions are all examples of particles that students might meet at 11–14. But these terms are often used incorrectly in the media and everyday language leading to students of all ages being confused as to which is the correct term to use.

Students should understand that:
- Particles can be atoms, molecules or ions.
- Atoms are single neutral particles.
- Molecules are neutral particles made of two or more atoms bonded together.
- An ion is a positively or negatively charged particle.
Ideas for your classroom
The idea of the world being made of tiny particles is an ancient one. You could start the exploration of atoms with the ideas of Democratus (400 BC), who believed that all matter in the universe was made up of tiny, indivisible, solid objects. He called these objects atoma or ‘indivisible units’. At the start of the 19th century Dalton found evidence to support Democratus’ theory and proposed atoms to be solid spheres. Different spheres made up the different elements.
One of the key problems for students learning about atoms, is that atoms are small. Really, really small. This makes it difficult for students to conceptualise atoms as they cannot be seen, or touched, or investigated directly. A good starting point to introduce atoms and illustrate their small size is to ask students to break up a piece of graphite (the element carbon) into as many small pieces as they can. No matter how many pieces the students break the graphite into, they will never get a single carbon atom. You can challenge higher attaining students to measure the size of an individual atom using this experiment from Practical physics.
When atoms combine, molecules are formed. For a few elements, when atoms of that element combine, a molecule of that element is formed eg H2 and O2. When atoms of some different elements combine, a molecule of a compound can form, eg H2 O. How to teach elements and compounds, in the 11–14 series, describes different strategies for teaching elements and compounds and the common misconceptions students may hold.
Particle diagrams can be used to help the students visualise the difference between an atom, a molecule of an element and a molecule of a compound. In fact even Dalton in the 1800s proposed a series of diagrams to represent the elements and compounds known at the time. Use of colour helps to distinguish between the atom types further. Venn diagrams help students organise their understanding of the different particle types, as described in Atoms, elements, molecules, compounds and mixtures.
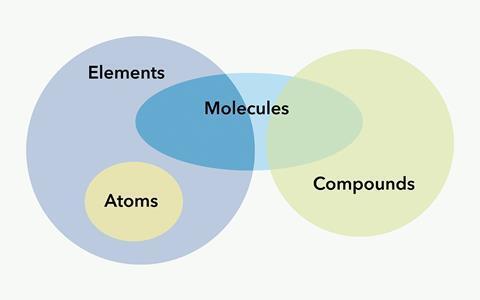
An atom or a molecule can lose or gain electron(s) to form an ion. At this level students only need to know that an ion is a positively or negatively charged particle. However it may be worth introducing students to the electron at this point. When an atom/molecule gains negatively charged electron(s), a negative ion is formed. When an atom/molecule loses negatively charged electron(s), a positive ion is formed. This latter point is something students often struggle with later on in their studies. Introducing the electron now, before students meet the other sub-atomic particles, can help to embed the idea that the loss of electrons results in a positively charged ion, and may help reduce confusion later on.
Owing to the interweaving of the terms atom, ion and molecule when describing the different particles, it is unsurprising that students get confused. Using games and an element of competition can be helpful to bring some variety to the necessary student practice. One such game is based on the classic Connect 4 game. You can download instructions, an example grid and game cards below.
Common misconceptions
As the students develop their understanding of chemical bonding further, it is common for students to refer to ionic compounds as molecules or to refer to intermolecular forces when explaining properties of ionic compounds. To avoid these misconceptions, it is important to introduce, and emphasise, the correct use of the terms ion and molecule from early on in a student’s chemical studies.
A molecule is a neutral particle, composed of a set number of atoms bonded together. The particle of the substance is the molecule, rather than the atoms that make up the molecule. By contrast, ionic compounds are made up of an indeterminate number of ions, in a fixed ratio. The particle of the ionic substance remains the ion. Using hands-on models can help students with these tricky concepts – eg TIMSTAR MO84200 for molecules and Molymod MKO-127-27 for ionic structures. You can further explore the use of chemical models and their limitations in Using molecular models and in the 7 simple rules to for science teaching series.
Other misconceptions students may hold are discussed in Beyond appearances: Students misconceptions about basic chemical ideas, including that atoms share the properties of the bulk material and that molecules have different properties in different states.
Progression to 14–16
At 14–16, students are introduced to sub-atomic particles and how these define the nature of atoms and ions. Students then go on to study the difference between the nature of the forces that exist between atoms, molecules and ions, which they use to explain the physical properties of ionic and covalent compounds.
The resource, Why do atoms form ions allows students to assess their understanding of atoms, ions and ionic compounds and enables the teacher to identify any misconceptions.
Take-home points
- Particles can be atoms, molecules or ions.
- The term molecule is often used incorrectly to refer to any type of chemical compound. A molecule is a neutral particle made of two or more atoms bonded together.
- Take care with your own language, especially when referring to compounds formed during chemical reactions. Make the distinction between each particle type explicit. Give students the opportunity to organise their understanding of the different particle types with Venn diagrams.
- A good understanding of the different particle types will help students when they study structure and bonding at 14–16.
