광자는 파동의 입자이다. 이는 파동의 주파수에만 의존하는 고정 된 양의 에너지이다. 광자의 에너지는 방정식 E = h f로 주어지며, 여기서 E는 광자의 에너지, h는 판 상수, f는 파동의 주파수이다. 광자는 에너지의 패킷으로 간주. 상대성 이론의 발달로 파동에도 질량이 있다는 것이 발견되었다. 이것은 파동이 물질과의 상호 작용에서 입자처럼 행동하기 때문이다. 그러나 광자의 나머지 질량은 0이다. 광자가 빛의 속도로 움직일 때, 그것은 E / C 2 의 상대론적 질량을 가지며, 여기서 E는 광자의 에너지이고 C는 진공 속의 빛의 씨앗이다.
광자와 전자의 차이는?
• 광자는 에너지의 패킷이고 전자는 질량이다.
• 광자는 정지 질량을 가지지 않지만 전자는 정지 질량을 갖는다. • 광자는 빛의 속도로 움직일 수 있지만 전자의 경우 빛의 속도를 얻는 것은 이론적으로 불가능하다. • 광자는 더 많은 웨이브 속성을 표시하지만 전자는 더 많은 입자 속성을 표시
Electron
The atom comprises three particles: electrons, protons and neutrons. The electron is a subatomic particle that carries a negative charge. Electrons are bound to the atom’s nucleus in, what is called, sub-shells. There are free electrons, too, which are not bound to any atom. Electrons are represented as e or ß. As mentioned earlier, electrons are negatively charged and is measured in Coulomb (the charge of an electron is given as

Among the three constituents of an atom, electrons have the lowest mass. The mass of one electron is around 1,800 times less than a proton. If calculated, it comes to 9.10938356 × 10-31 kilograms.
Electron Wavelength
Louis de Broglie first measured the wavelength of an electron. He used the concepts of Einstein’s formula and Planck’s constant to calculate it. The equation which measures the wavelength of an electron is:
Where,
λ is the wavelength.
h is Planck’s constant.
p is the momentum of the electron.
Photon
Photon is a type of elementary particle with a zero rest mass and moves with the speed of light in a vacuum. A photon is the “quantum of electromagnetic radiation”. In other words, it is the smallest and the fundamental particle of electromagnetic radiation. A photon has no mass, no electric charge, and is a stable particle. These particles possess wave-particle duality. It has 2 polarization states. Photons do not obey Pauli’s Exclusion Principle. Photons are emitted in natural processes. For example, during a nuclear, molecular and atomic transition to a lower level, various photons with different energy levels are emitted, ranging from radio waves to gamma rays. They are also emitted when a particle and its antiparticle are annihilated. The equation E = pc relates the energy and momentum of a photon.
where p is the magnitude of vector p, this comes from the equation
Photon’s energy and momentum depend on its frequency(v) and wavelength(λ).
Photon also has a spin angular momentum quantity that does not depend on its frequency.
Light, for example, is electromagnetic radiation. Photons can be said to be the fundamental particles of light. It can be said that light is carried over space by photons.
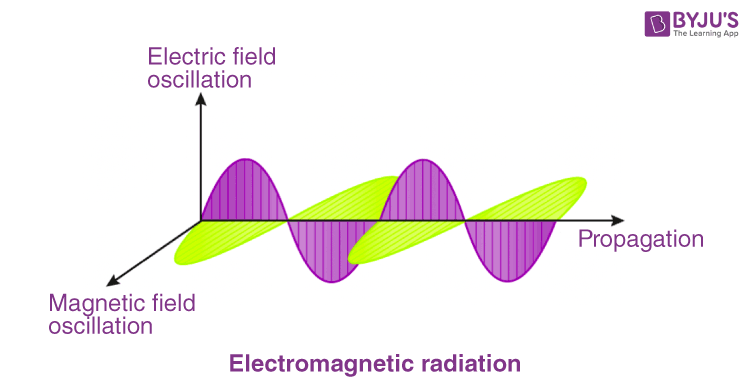
It must be remembered that photons describe the properties of the particles of a wave and not the overall properties of the wave itself. Also, as the photon is essentially nothing but energy, it has no mass whatsoever.
Difference Between Photon and Electron
Photons and electrons are both fundamental particles, the former of electromagnetic waves and the latter of atoms. Together, they can cause the flow of electricity. However, they are different in many aspects:
| Electron | Photon |
| It is a matter. | It is energy. |
| It has mass. | It has no mass. |
| It carries a negative charge. | It doesn’t carry any charge. |
| The speed of an electron can be zero or anything under the speed of light. It is impossible for an electron to achieve the speed of light. | Photons travel at the speed of light. |