근육에서도 다양한 생리 활성물질이 분비된다고 하는데 이들 물질을 총칭하여 ‘마이오카인’(myokines)이라 한다. 현재까지 발견된 것만 30종 이상이라 한다. 물론 일부를 제외하고 어떤 일을 하는지 충분히 밝혀지진 않았다. 하지만 연구가 진행된 물질 중에는 여러 가지 질병을 막는데 효과가 있다는 사실이 밝혀지고 있다.
골격근이 체중에서 차지하는 비중은 약 40~50%라고 한다. 종래에는 몸을 움직이기 위한 조직이라고만 생각해 왔으나, 호르몬을 생성한다는 것이 알려지며 주목을 받게 되었다. 우리몸의 40~50%를 차지하는 만큼 근육이 최대의 내분비기관이라 봐도 좋을지도 모를 일이다.
주요 마이오카인과 그 기능
| 사이토카인 | 주요 기능 |
| -myostatin,IL-6,IL-7,LIF,decorin | 골격근의 발생・비대・손상으로부터 재생 제어 |
| -BDNF,IL-6,adiponectin,irisin (fibronectin type III domain containing protein 5) |
AMPK 의존성 지질대사, PGC-1α 활성화(?) |
| -IGF-1,FGF-2 | 인접 골조직의 강화 |
| -FSTL-1(follistatin related protein 1) | 혈관 내피기능의 조절 |
| -CTRP-15 | 간장 오토파지의 제어 |
| -IL-1RA,IL-10 | 염증제어(?)・IL-6 분비조절(?)(피드백) |
| -SPARC | 화학 발암의 제어(?) |
| -CXC 케모카인(CXCL1/5) | 근섬유 아세포의 유주(?) |
| -TNFα,IL-1β,CSF3 | 염증제어(?) |
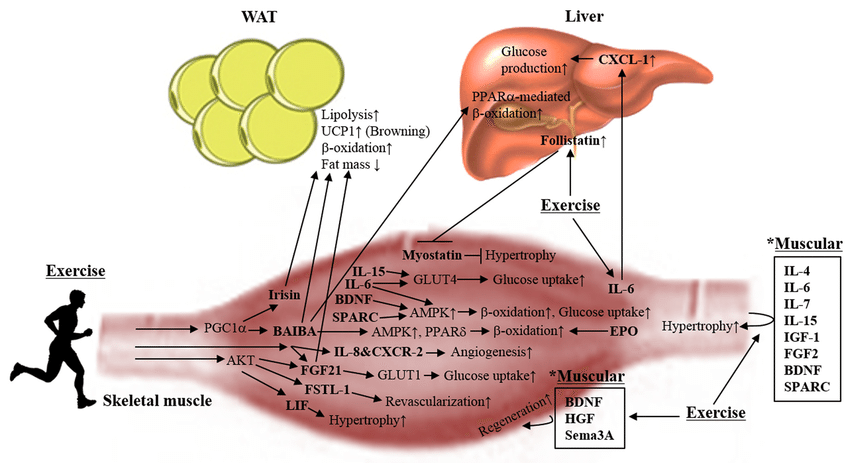
운동을 통해 조절되는 마이오카인과 대사물은 내분비 효과를 갖는다. 마이오카인은 오토크린과 파라크린 효과 외에도 지방 조직, 간, 내장, 뇌, 췌장, 뼈, 각종 면역 세포, 종양 등에도 작용한다. 운동은 주로 마이오카인과 대사물의 방출을 자극한다(그림의 검은색 글자. BAIBA, β-아미노 이소부티르산 등). 다만 미오스타틴과 키뉴레나인의 경우는 규칙적인 운동 후에 감소된다고 한다(빨간색 글자). Hoffmann C 외(2017), Skeletal Muscle as an Endocrine Organ: The Role of Myokines in Exercise Adaptations)
위의 표 및 그림에서와 같이 다양한 마이오카인이 확인되고 그 기능에 대한 연구가 이루어지고 있다고 한다. 이들 마이오카인은 직접적으로 골격근의 기능성을 보호하고 그 운동능력을 향상시키는 역할을 한다. 각종 마이오카인이 에너지 대사, 증식, 혈관신생, 염증 과정, 세포 외 기질 등을 조절하는 역할을 함으로써 골격근의 적응 과정을 조절해 준다. 나아가 마이오카인은 인체 전반에 작용하여 체중 조절, 염증, 인슐린 민감성, 종양 성장 억제, 인지 기능 향상 등의 내분비 기능에도 관여한다.
현재 그 효용이 알려진 마이오카인을 몇가지 들자면, 'SPARC' 'IL-6' 'FGF-21' '아디포넥틴' '아이리신' 'IGF-1' 등이 있다. 이들 가운데는 하나의 질병 뿐만 아니라 여러 질병에 걸쳐 효과를 보이는 것도 있는데, 여기서는 위에 든 몇가지에 대해 그 효능을 중심으로 간략이 정리해 둔다.
가장 대표적인 호르몬 "SPARC"은 대장암의 암세포를 "자살(아포토시스)"시키는 작용이 있는 것으로 알려져 있다.
"원래 운동을 좋아하는 사람은 대장암에 잘 걸리지 않는다고 한다. 한편 SPARC가 대장암 억제인자라는 것은 알려져 있었지만, 근육에서 분비되는 것으로 판명되면서 운동과 SPARC 간의 밀접한 관계가 주목 받게 되었다."(大妻女子大学 家政学部 高波嘉一교수)
다음으로 "IL-6". 운동시 혈장내 IL-6의 수치가 100배까지 높아지기도 하는데, 이 호르몬은 인슐린 분비를 촉진시켜 체내의 당분을 가져가고 간에서 지방 분해를 촉진한다. 따라서 비만과 당뇨병을 억제하는 효능이 있다. 또한 항염증작용도 있어 면역기능 조절효과를 통해 노화, 심혈관질환, 암 등의 발생을 억제하며, 근 성장을 자극하고 혈관 신생에도 작용한다는 사실이 밝혀지고 있다.
"FGF-21'도 간에서 지방을 분해한다. 또한 지방세포에 작용하여 연소시키는 작용을 하는 것으로 보인다고 한다. 간경변으로 이어지게 되는 지방간을 개선하는 역할을 하는 마이오카인이다.
'아디포네틱'은 원래 지방세포 및 간에서 분비되는 것으로 알려져 있으며, 지질을 분해하는 작용이 있다. 이것은 당뇨병과 이상 지질에서 오는 동맥경화를 방지하는 효능이 있는데, 10년 전 근육에서도 분비되는 것으로 밝혀졌다. 또한 최근 연구에서 인슐린 감수성을 높이고, 항염증, 혈관 보호, 간에서의 당 생성 억제, 근육의 포도당 흡수 및 지방상의 산화 증가, 염증성 사이토카인 억제 및 항염증성 사이토카인의 증가, 내피세포의 기능 손상 억제 등의 기능이 있다고 한다.
운동생화학이 전공인 쓰쿠바대학 체육계의 征矢英昭교수는 말한다. "아직 동물실험 단계이지만, 마우스 휠 돌리기 운동에 의해 아디포넥틴이 뇌로 들어가 해마의 신경 신생을 촉진한다는 보고가 제출되었다. 스트레스와 우울증, 인지증이 되면 신경이 신생되지 않고 해마가 위축되어 버리는데, 운동을 함으로써 해마에 새로운 신경이 늘어날 가능성이 있다."
한편 하버드대학 연구팀은 "아이리신'을 주목하고 있다. 아이리신은 혈관을 타고 지방조직으로 이동해 백색지방을 갈색지방으로 바꿔 열 생성을 유도한다. 이른바 지방을 태운다는 의미이다. 또 동물실험이긴 하지만, 하버드 대학의 논문에서는 아이리신이 뇌에도 영향을 미친다고 한다. 자세한 것은 불명이지만, 근육에서 분비된 아이리신이 뇌에 들어가면 인지 기능을 개선하는 BDNF(brain-derived neurotrophic factor, 뇌유래 신경성장인자)의 발현에 효과가 있다는 것이다. 인지기능의 저하 가능성이 높아지는 노령인구에서도 근 기능 향상이 중요하다는 점을 시사해 준다.
"IGF-1"도 신경에 작용한다고 볼 수 있다. 이 호르몬은 원래 근육과 뼈의 성장 촉진에 필수적인 물질로 알려져 있었다. 하지만 이 물질이 신경세포를 만들어 시냅스의 결합, 심지어는 혈관신생을 촉진하는 것으로 밝혀졌다고 한다. 실제로 2010년 한 연구에서 IGF-1이 뇌 혈관을 통해 뇌신경에 작용하는 것으로 확인되었다.
"가벼운 운동에 의해 뇌신경이 활성화되면 신경에 가까운 혈관이 열리고 혈액에 의해 산소와 IGF-1이 실려 운반된다. 그 때, IGF-1은 산소와 함께 작은 혈관의 구멍을 통과하여 뇌에 유입된다. 그 결과, 신경의 활동을 강화하고 알츠하이머병의 원인물질 중 하나인 베타 아밀로이드를 감소시키는 효과가 있다"고 한다.
항염증작용에 의한 면역기능 조절, 지방분해 활성화, 조직 신생, 특히 뇌신경 활성화 등 다양한 작용으로 인체 생리를 활성화하는 등 폭넓게 작용하므로써, 각종 질병의 예방에 중요한 의미를 갖는다고 볼 수 있다.
이상에서와 같이 마이오카인은 여러 질병에 효과가 있음을 알 수 있는데, 물론 예외도 있다. '마이오스타틴'이라는 물질이 그것이다. "마이오스타틴은 근육의 성장을 억제하는 작용이 있는 것으로 알려져 있다. 실제로 마이오스타틴의 작용을 억제시킨 쥐가 근육이 빵빵해 졌다는 실험보고가 있고, 선천적으로 마이오스타틴이 적어서 어릴 때부터 근육질인 사람도 있다고 한다. 그러나 발상을 전환하고 이를 잘 제어하는 약물을 개발할 수 있다면, 노쇠한 노인이 걸을 수 있게 될지도 모른다"(도쿠시마 대학 첨단효소학연구소의 親泊政一교수)
현재 마이오카인 연구에 제약업체들도 참가하여 뜨거운 열기를 보이고 있지만, 불행히도 약으로 사용되는 것은 아직 없다. "예를 들어, IL-6가 신약으로 개발되기도 하였으나, 항체의약품처럼 비싼 약이 될 가능성도 있다."고 한다. 하지만 마이오카인은 스스로의 힘으로 몸 안에서 만들 수가 있다. 값 비싼 약물이 개발되기를 기다리는 것보다 싸게 스스로 늘리는 방법을 취하면 좋을 것이다.
이처럼 근육에서 생성되는 마이오카인은 인체의 항상성 유지를 중심으로 인체생리에 중요한 역할을 함을 알 수 있다. 면역력 제고는 물론 지방의 분해에 작용하여 성인병 예방에도 효과가 있다. 또한 세포 활성을 높여 콜라겐 생성을 촉진함으로써 피부에 탄력성을 가져다 준다. 뿐만 아니라 뇌신경 활성화 작용으로 인지증 예방에 유효할 것이다. 또한 마이토카인은 암 증식 억제기전의 일부를 담당하는 것으로 이해되고 있으며, 특히 SPARC 등 일부는 암 증식 억제 효과가 있다고 한다. 따라서 운동을 통해 근력을 향상시켜 마이오카인 분비를 촉진토록 할 필요가 있다.
결론은 역시 운동이다. 노화에 따른 근육의 양과 힘, 기능이 약화되는 현상을 늦추기 위해서는 무엇보다 운동이 중요하다는 것이다.
마이오카인 분비 촉진을 주목할 경우, 일반적인 운동과는 좀 다른 방식을 권한다. 일정한 시간 매일매일 운동 하기 보다는 강도나 횟수를 달리 하는 불규칙한 운동, 근육의 반응에 충격이라 느껴질 정도의 상대적으로 좀 더 격한 운동, 그리고 운동 사이사이의 충분한 휴식 등이 강조되기도 한다. 특히 운동에서 핵심/코어 근육의 단련이 강조된다. 모두 마이오카인의 분비를 촉진하는데 맞춰진 방법들이다. 예를 들자면 왕복달리기와 같은 운동이 대표적이다. 이를 잘 감안하면 필요한 운동을 유추하여 실행할 수 있을 것이다. 한편 자전거 운동에 주목하기도 한다. 자전거타기는 다리를 포함한 전신운동이지만, 마이오카인 분비가 특히 많다고 하는 허벅지를 크게 움직이는 특징이 있기 때문일 것이다.
Role of Myokines in Regulating Skeletal Muscle Mass and Function
Loss of skeletal muscle mass and strength has recently become a hot research topic with the extension of life span and an increasingly sedentary lifestyle in modern society. Maintenance of skeletal muscle mass is considered an essential determinant of muscle strength and function. Myokines are cytokines synthesized and released by myocytes during muscular contractions. They are implicated in autocrine regulation of metabolism in the muscle as well as in the paracrine/endocrine regulation of other tissues and organs including adipose tissue, the liver, and the brain through their receptors. Till date, secretome analysis of human myocyte culture medium has revealed over 600 myokines. In this review article, we summarize our current knowledge of major identified and characterized myokines focusing on their biological activity and function, particularly in muscle mass and function.
Introduction
The muscle is a tissue composed of cells or fibers that produce force and movement of the body. They are primarily responsible for maintaining and changing body position, locomotion as well as the movement of internal organs. Different types of muscles perform different functions according to their location and type. Skeletal muscles are one of the most dynamic tissues involved in voluntary contraction according to command (Frontera and Ochala, 2015; Noto and Edens, 2018). They comprise approximately 40% of the total body weight (Frontera and Ochala, 2015; Noto and Edens, 2018). In contrast, cardiac and smooth muscles are associated with involuntary contraction without awareness (Frontera and Ochala, 2015; Hafen and Burns, 2018; Noto and Edens, 2018). Smooth muscles are found throughout the body and tightly regulate many of the body subsystems implicated in maintaining survival (Hafen and Burns, 2018).
Myokines are cytokines or peptides synthesized and released by myocytes in muscle tissue in response to muscular contractions (Pedersen et al., 2007). The term “myokine” was first introduced by a Swedish scientist, Bengt Saltin, in 2003 (Pedersen et al., 2003). Myokines are implicated in the autocrine regulation of metabolism in muscles as well as in the para/endocrine regulation of other tissues and organs including the adipose tissue, liver, and brain (Carson, 2017) through their receptors. Since myostatin was first identified as a myokine in 1997, secretome-based analysis of human myocyte culture medium has revealed over 600 myokines till date (Gorgens et al., 2015). However, the majority of these myokines are still not sufficiently characterized. Only few of them have been studied for their biological activity and function and have provided some clear evidence as being released directly from muscle contraction. Moreover, studies potentially associated with muscle atrophy barely exist. Understanding the biological and physiological roles of these myokines in skeletal muscle atrophy or weakness is important and valuable to find novel targets for therapeutic intervention.
In this review, we summarize our current knowledge focusing on myokines released directly by muscle contraction and their potential roles associated with skeletal muscle mass and function.
Myostatin
Myostatin, growth differentiation factor 8, was the first identified myokine in 1997 by Se-Jin Lee and his colleagues (McPherron et al., 1997). It is encoded by the myostatin gene and is known as a highly conserved member of the TGF beta protein family (McPherron et al., 1997). It is abundantly expressed in skeletal muscles, but is also expressed to a lesser extent in cardiac muscles and fat tissues (McPherron et al., 1997; Sharma et al., 1999). Myostatin levels in the plasma of healthy young men has been shown to significantly decrease within 24 h post-exercise when compared to pre-exercise and has also been shown to positively correlate with plasma IL-6 (Kazemi, 2016). In contrast, serum myostatin has been shown to increase in patients with spinal cord injury after aerobic exercise (Han et al., 2016). Although heavily contradicting reports exist on both sides, circulating myostatin shows an obvious increase in females rather than males during sarcopenia (Bergen et al., 2015), and a decrease in cancer-cachexia (Loumaye and Thissen, 2017) and generic neuromuscular disease patients (Awano et al., 2008; Anaya-Segura et al., 2015; Burch et al., 2017).
The effects of myostatin are mediated through the activin type IIB receptor (ActRIIB), which is expressed ubiquitously (Pistilli et al., 2011; Amthor and Hoogaars, 2012). The downstream mediators of myostatin, Smad2 and Smad3, are phosphorylated and form a complex with Smad4. This complex in turn stimulates FoxO-dependent transcription and regulates the transcription of genes associated with the proliferation and differentiation in skeletal muscle precursor cells as well as protein degradation pathways (such as the ubiquitin-proteasome processes, and autophagy) in mature myofibers (Burks and Cohn, 2011; Han et al., 2013). In addition, myostatin-mediated Smad signaling activation inhibits protein synthesis in muscle tissues by suppressing the Akt-mediated mTOR signaling pathway (Han et al., 2013). Functionally, myostatin is a negative regulator of muscle growth thereby leading to inhibition of myogenesis through muscle cell differentiation and growth (McPherron et al., 1997). Animals blocking myostatin activity with substance show significantly increased muscle mass (myofiber hypertrophy rather than hyperplasia) (Morvan et al., 2017). In myostatin-knockout mice, the muscle mass is approximately twice increased compared to that in normal mice (McPherron et al., 1997). In humans, individuals with mutations in both copies of the myostatin gene showed significantly increased muscle mass and muscle strength compared to that observed in normal individuals (Schuelke et al., 2004). Growing evidence indicates that increasing myostatin and its analog activin A contribute to the incidence of muscle atrophy (Morvan et al., 2017). Thus, myostatin is considered a promising target molecule for the treatment of muscle wasting. In the past two decades, several agents, such as follistatin (myostatin antagonist), and selective antibody-based approaches targeting ActR-IIB, myostatin, and activin A were developed to antagonize/suppress myostatin signaling. These molecules were evaluated under various pathological conditions such as muscular wasting or atrophy. For example, a myostatin antibody, MYO-029/stamulumab, was tested in broad muscle dystrophic models, including Becker’s muscular dystrophy (BMD) and facioscapulohumeral dystrophy, but failed to show clinical efficacy in elevating muscle strength (Leung et al., 2015). Overexpression of the follistatin isoform, FS344, using an AVV vector showed improved ambulation in patients with BMD and inclusion body myositis (Al-Zaidy et al., 2015; Mendell et al., 2015; Mendell et al., 2017). However, so far none of these treatments have proven to be clinically sufficient as shown in Table 1 (Cohen et al., 2015; Mariot et al., 2017). There are still obstacles (such as lack of target specificity and potential clinical toxicities) to overcome for their use in human patients. In addition, a recent study showed that activin A prominently regulates muscle mass in primates than does myostatin in rodents (Busquets et al., 2012; Cohen et al., 2015), suggesting that targeting myostatin alone may not be sufficient to treat muscle atrophy in humans.
Irisin
Irisin is a cleaved form of Fibronectin type III domain-containing protein 5 (FNDC5), which was simultaneously discovered by two independent groups in 2002 (Teufel et al., 2002; Colaianni et al., 2014) It was first reported as a potential mediator of the beneficial effect of exercise (Raschke et al., 2013b). Initially, the expression of PGC1α in muscle stimulates FNDC5 expression, which drives brown fat-like development of white fat cells named beige cells and increases thermogenesis (Bostrom et al., 2012). Although exercise-induced increase in the level of irisin in the blood is heavily debated (Pekkala et al., 2013), many reports have continuously shown an increase in FNDC5 mRNA expression upon exercise in rodent models (Dun et al., 2013; Roberts et al., 2013) and humans (Huh et al., 2012; Lecker et al., 2012), thus triggering renewed interest in exercise-induced myokines. In line with these observations, expression of mitochondrial-specific transcription factors, such as PGC-1α and mitochondrial transcription factor A, increases in C2C12 myotubes exposure to recombinant irisin for 24 h. They are all involved in elevated mitochondrial content and oxygen consumption (Vaughan et al., 2015). Moreover, irisin and myostatin are inversely secreted from skeletal muscles after physical exercise (MacKenzie et al., 2013), thereby suggesting its potential myogenic role. Reza et al. reported that irisin induced skeletal muscle hypertrophy and attenuated denervation-induced atrophy by activating IL-6 signaling in rodents (Reza et al., 2017). The effects of irisin on hypertrophy were shown to be established by the activation of muscle satellite cells and elevation of protein synthesis (Reza et al., 2017). This study substantially opened up potential research avenues on irisin with respect to muscle atrophy. Moreover, a latest study showed that circulating irisin levels were lower in women with postmenopausal sarcopenia when compared to those with pre-sarcopenia and that they negatively correlated with the quadricep cross-sectional area (Park et al., 2018), suggesting that irisin may also function as a potential pro-myogenic factor in human pathological conditions. Further studies are needed to reveal the biological effects of human irisin and the underlying mechanism in human skeletal muscles.
IL-6
Interleukin 6 (IL-6) was identified in 2000 and is the most studied myokine (Steensberg et al., 2000; Pedersen and Febbraio, 2008). It is secreted from muscles into the blood vessel in response to muscle contractions (Pedersen and Febbraio, 2008), by which skeletal muscles communicate with central and peripheral organs (Pedersen et al., 2003). The circulatory level of IL-6 is affected by both the duration and intensity of muscle contraction in humans (Steensberg et al., 2000; Helge et al., 2003). Interestingly, IL-6 is highly produced and released after post-exercise while insulin action is enhanced. However, IL-6 is also associated with obesity and insulin resistance (Pedersen and Febbraio, 2008). IL-6 has an insulin-like effect on glucose metabolism. IL-6 increases insulin-stimulated glucose disposal in humans as well as glucose uptake and fatty acid oxidation in vitro through AMP-activated protein kinase and PI3K-Akt signaling pathways (Al-Khalili et al., 2006; Carey et al., 2006). Individuals with spinal cord injury (SCI) are prone to develop metabolic diseases due to the lack of exercise-related IL-6 response, suggesting that IL-6 plays a pivotal role in regulating glucose homeostasis (Kouda et al., 2012).
On the other hand, the role of IL-6 on muscle atrophy seems to be a negative effect rather than a beneficial effect. Increased circulating angiotensin II (AngII) reduces lean body mass in chronic kidney disease. In mice, AngII infusion resulted in increased circulating IL-6 and its hepatic production, suggesting that AngII-induced inflammation might be a trigger for muscle loss (Zhang et al., 2009). In contrast, AngII-induced muscle atrophy was suppressed in IL-6-deficient mice (Zhang et al., 2009). IL-6 is overproduced in patients with Duchenne muscular dystrophy and in muscles of the mdx animal model. Inhibition of IL-6 activity with an interleukin-6 receptor (Il-6r) neutralizing antibody attenuates the dystrophic phenotype, severe muscle degeneration, inflammation, as well as accumulation of non-functional fat and fibrotic tissues (Wada et al., 2017). In addition, pharmacological inhibition of IL-6 activity in mdx male mice inhibits anti-inflammatory responses and improvement in muscle repair (Pelosi et al., 2015). Therefore, inhibition of IL-6 might be beneficial for preventing muscle loss.
Brain-Derived Neurotrophic Factor
Brain-derived neurotrophic factor (BDNF) is the second member of the neurotrophin family of growth factors, which regulates neuronal survival, plasticity, growth, and death through tropomyosin-related kinase receptor B (TrkB). It was for the first time purified from pig brain in 1982 (Barde et al., 1982). After 11 years, the BDNF gene was identified by two independent groups (Metsis et al., 1993; Binder and Scharfman, 2004). Initially, BDNF has been studied mostly in relation with nervous system development and function (Clow and Jasmin, 2010). However, the expression of several neurotrophin receptors is identified in skeletal muscles, thus implicating the certain role of BDNF. Indeed, Chevrel et al. (2006) reported that BDNF is differentially expressed in skeletal muscles according to physiological or pathological conditions. In adult skeletal muscles, BDNF is also expressed in muscle satellite cells (Mousavi et al., 2004) and is upregulated in muscle injury followed by the activation and proliferation of satellite cells, suggesting that BDNF might play an important role in mediating the satellite cell response to muscle injury (Omura et al., 2005). Jasmin et al. showed that BDNF substantially regulates satellite cell differentiation and skeletal muscle regeneration, by using BDNF null and muscle-specific BDNF KO mice (Clow and Jasmin, 2010). These results indicate that BDNF might be involved in the regulation of damaged muscles. Although there are many studies associated with the role of BDNF in muscle development and function, there is no clear evidence indicating that it is a myokine. In fact, the effect of muscle contraction on circulating BDNF levels is controversial. Some studies have reported no change in serum BDNF right after either acute or chronic exercise. On the other hand, several studies have shown that circulating BDNF increases with physical exercise (Ferris et al., 2007; Yarrow et al., 2010; Pereira et al., 2018). In skeletal muscle cells, BDNF mRNA expression is increased by contraction and increased fat oxidation through activation of AMP-activated protein kinase (Matthews et al., 2009). Overall, these studies suggest that muscle-derived BDNF is important for regulating muscle regeneration right after muscle injury. However, many key questions on the biological functions of BDNF in skeletal muscles remain unresolved. A major issue would be to elucidate the mechanism by which BDNF regulates satellite cell differentiation and skeletal muscle regeneration, and in which BDNF substantially recover muscle strength and function. Manipulating BDNF may thus represent an important therapeutic tool for alleviating dystrophic muscle atrophy.
IL-15
Interleukin-15 (IL-15) is a cytokine with a structure similar to interleukin-2 (IL-2). It was discovered by two different research groups in 1994 and was characterized as a T cell growth factor (Steel et al., 2012). Later on, several studies showed that IL-15 is accumulated in the muscles as a result of regular exercise training, indicating that it is a myokine (Pedersen, 2011; Tamura et al., 2011; Brunelli et al., 2015). Moreover, IL-15 mRNA expression is upregulated along with myoblast differentiation (Pedersen and Febbraio, 2008). Supportively, several studies showed that exogenously treated IL-15 or IL-15 overexpression promotes myoblast differentiation and increases muscle mass in the mouse C2 skeletal myogenic cell line (Quinn et al., 1995, 2002). In rats with cancer cachexia, IL-15 treatment attenuates skeletal muscle wasting by suppressing protein degradation through inhibition of the ATP-dependent ubiquitin proteolytic pathway (Carbo et al., 2000). IL-15 administration was found to improve diaphragm strength with increased muscle fiber cross-sectional area and decreased collagen accumulation in dystrophic mdx mice (Harcourt et al., 2005). In contrast, systemic infusion of IL-15 induces muscle atrophy in skeletal muscles of rodents (Pistilli and Alway, 2008). IL-15 treatment increased the glucose uptake in skeletal muscle cells via activation of the Jak3/STAT3 signaling pathway (Krolopp et al., 2016) or the AMPK signaling pathway (Gray and Kamolrat, 2011). In addition, Quinn L et al. and coworkers reported that IL-15 transgenic mice exhibited increased fat oxidation, energy expenditure and running endurance even with lower muscle mass compared to that in wild type mice. Interestingly, these mice also expressed troponin I and myosin heavy chain mRNA isoform indicating the conversion of muscles to a more oxidative phenotype (Quinn et al., 2013; Chalkiadaki et al., 2014). Collectively, the above controversial reports indicate that IL-15 acts differently according to the normal and pathological conditions. Thus, further studies should be focused on clarifying the different factors influencing the varying roles of IL-15 between different physiological conditions.
Myonectin (CTRP15)
Myonectin is a myokine belonging to the C1q/TNF-related protein (CTRP) family, and was discovered by Seldin et al. (2012). It is a novel nutrient-responsive myokine secreted from skeletal muscles (Seldin et al., 2012; Peterson et al., 2014). Myonectin is released into the blood stream by muscular contraction, and is functionally similar to insulin as it promotes fatty acid uptake into cells by increased expression of fatty acid transport genes (CD36, FATP1, Fabp1, and Fabp4) (Seldin and Wong, 2012; Seldin et al., 2012). In the mouse liver and cultured hepatocytes, recombinant myonectin treatment suppresses starvation-induced autophagy by inhibiting LC3-dependent autophagosome formation, p62 degradation, and other autophagy-related genes expression. Furthermore, the ability of myonectin to suppress autophagy is abolished by inhibition of the PI3K/Akt/mTOR signaling pathway (Seldin et al., 2013). Autophagy is considered to be a mechanism that induces muscle atrophy (Bonaldo and Sandri, 2013). In addition, the PI3K/Akt signaling pathway is involved in anabolic responses in the body. Therefore, these observations indicate that myonectin may play an important role in increasing muscle mass by elevating protein synthesis and inhibiting protein degradation. On a related note, mitochondrial content in muscles is an important determinant for muscle type and function. Myonectin is remarkably increased following depletion of mtDNA and increases glucose uptake and fatty acid oxidation through activation of the AMPK signaling pathway in rat skeletal myocytes (Park et al., 2009; Lim et al., 2012). Interestingly, oxidative, slow-twitch muscle fiber types express a higher level of myonectin than does glycolytic, fast-twitch muscle fiber types, suggesting that it may be involved in mitochondrial biogenesis and sensing cellular energy state (Seldin and Wong, 2012). However, there is no study associated with its biological function and mechanism on muscle mass and muscle mitochondrial biogenesis in normal physiology and in the diseased state.
Decorin
Decorin is small leucine-rich proteoglycan identified as a myokine by Kanzleiter et al. (2014). It is secreted in skeletal muscles during muscular contraction and plays an important role in muscle growth. Decorin directly binds to and inactivates myostatin (a potent inhibitor of muscle growth) in a zinc-dependent manner, and inhibits its anti-myogenic effects (El Shafey et al., 2016). In vivo over-expression of Decorin in murine skeletal muscles promotes expression of the pro-myogenic factor Mighty (Marshall et al., 2008). Mighty is ubiquitously expressed but appears to be negatively regulated by myostatin in skeletal muscles. Decorin overexpression increases the expression of Myod1 and follistatin, whereas it reduces the muscle-specific ubiquitin ligases atrogin1 and MuRF1 (Marshall et al., 2008). Thus, Decorin might act as a myogenic factor and might be a possible therapeutic target for the treatment of muscle wasting.
Fibroblast Growth Factor (FGF) 21
Fibroblast growth factors (FGFs) are signaling proteins with diverse biological functions in development and metabolism. FGFs are classified as para, intra, and endocrine according to their action manners. Paracrine FGFs mostly function as local signaling molecules in developmental processes whereas intracrine FGFs mainly serve as intracellular molecules in neuronal processes (Itoh and Ornitz, 2011). FGF21 functions as endocrinal hormone-like or local signaling molecules in metabolism. FGF21 does not have proliferative activity as other paracrine and endocrine FGFs family and is only associated with metabolism (Itoh, 2014). FGFs activate several intracellular signaling pathways including phosphatidylinositol 3-kinase (PI3K)/serine-threonine protein kinase AKT, signaling transducer and activator of transcription (STAT), mitogen activation protein kinase (MAPK), and phosphoinositide phospholipase C (PLC) γ (Itoh, 2014). Specifically, FGF21 acts through FGF receptor 1c with β-Klotho as a cofactor. Skeletal muscle-specific Akt1 transgenic mice showed skeletal muscle fiber hypertrophy with increasing Fgf21 expression in the muscle and in serum indicating that FGF21 plays an important role in regulating muscle mass (Izumiya et al., 2008). In addition, FGF21 expression is coupled to mitochondrial dysfunction and insults of various stresses in skeletal muscles. Autophagy deficiency and subsequent mitochondrial dysfunction elevates the level of FGF21 as a myokine, thereby protecting against diet-induced obesity and insulin resistance (Kim et al., 2013; Keipert et al., 2014). In cultured myoblasts, mitochondrial complex inhibitor treatment increased expression by promoting the binding of activating transcription factor 2 (ATF2) for the promoter region of the Fgf21 gene (Ribas et al., 2014). Moreover, in human brain vascular smooth muscle cells, FGF21 protects against angiotensin II-induced cerebrovascular aging by elevating mitochondrial biogenesis (Wang et al., 2016). All the above studies suggest that FGF21 may be potentially involved in switching muscle type and regulating mitophagy, thereby regulating muscle mass and function. Thus, targeting FGF21 might be an attractive approach to treat mitochondrial-based myopathy and muscle dysfunction.
Secreted Protein Acidic and Rich in Cysteine (SPARC)
Aoi et al. (2013) reported SPARC/osteonectin as a novel myokine, which is released from the skeletal muscles of both humans and mice after exercise, even though it was identified earlier (Aoi et al., 2013). Exercise-stimulated SPARC secretion was shown to inhibit colon tumorigenesis by enhancing apoptosis in colon cancer cells (Aoi et al., 2013). SPARC was also shown to be upregulated in inherited and idiopathic muscle wasting diseases such as Duchenne muscular dystrophy and congenital muscular dystrophy (Jorgensen et al., 2009). SPARC overexpression almost completely abolished myogenic differentiation in the muscle progenitor cell line, C2C12 (Petersson et al., 2013). Thus, SPARC may play a certain functional role in the repair of muscle damage in muscle satellite cells. However, very limited studies associated with the role of myokines are currently available. Further studies need to first determine and address the expression profile and role of SPARC in muscle development and regeneration. The underlying signaling pathways also need to be studied in detail.
Conclusion
Skeletal muscle atrophy is an emerging medical problem worldwide owing to increasing elderly populations and various classical reasons including genetic mutation, disease-derived cachexia, and accidents. However, although our understanding of molecular mechanisms regulating muscle atrophy/muscle weakness has substantially progressed, there is no specific treatment for muscle atrophy. Recently, a number of myokines have been identified through secretome analysis and some have proven to be very informative while searching for novel myokines (Raschke et al., 2013a; Hartwig et al., 2014; Grube et al., 2018). However, the majority of myokines are not still sufficiently characterized with regard to their biological activity and function. Only few myokines have been restrictively characterized (Figure 1) and their potential signaling pathways, implicated in muscle cell proliferation, differentiation, and growth in order to maintain muscle mass, muscle strength, and function, been identified (Figure 2). Therefore, it is important to better understand their precise role and function on skeletal muscles under normal physiological and pathophysiological conditions. Targeting novel myokines for either increasing or suppressing their functional activity in certain pathological states could be an attractive novel therapeutic tool for combating skeletal muscle atrophy.