가려움증은 습진이나 아토피 피부염 같은 질환을 더욱 괴롭게 만드는 요인이다. 많은 이들이 가려움증을 참지 못해 피부를 긁고, 이는 피부염을 더 악화시킨다.그러나 구체적으로 무엇이 어떻게 가려움증을 유발하는지는 그동안 정확히 규명되지 않았다. 전문가들은 대체로 피부에 생긴 염증이 동반되는 증상이나 면역 반응의 결과로 여겼다. 피부질환에 염증과 면역 반응을 억제하는 스테로이드 연고를 쓰는 이유가 여기에 있다.
미국 하버드대 의대 연구진이 쥐와 인간 세포 실험을 통해 호흡기관과 피부에 주로 서식하는 황색포도상구균(MRSA)이 피부의 신경세포를 자극해 가려움증을 유발한다는 사실을 밝혀내 국제학술지 ‘셀’에 발표했다. 황색포도상구균은 식중독의 원인 물질이기도 하다.연구진은 황색포도상구균이 염증과 상관 없이 긁고 싶은 충동을 일으키는 분자 연쇄 반응을 유발한다고 밝혔다.과학자들은 습진 환자의 피부에 황색포도상구균이 서식하는 경우가 많다는 사실을 오래전부터 알고 있었지만, 이 박테리아가 어떤 역할을 하는지는 분명하게 알지 못했다. 이번 연구로 그동안 피부 염증이 원인으로 생각했던 것이 사실은 피부에 염증이 생기면 피부 미생물의 균형이 깨지면서 황색포도상구균이 번성하는 경우가 많기 때문이라는 사실이 드러나게 됐다.
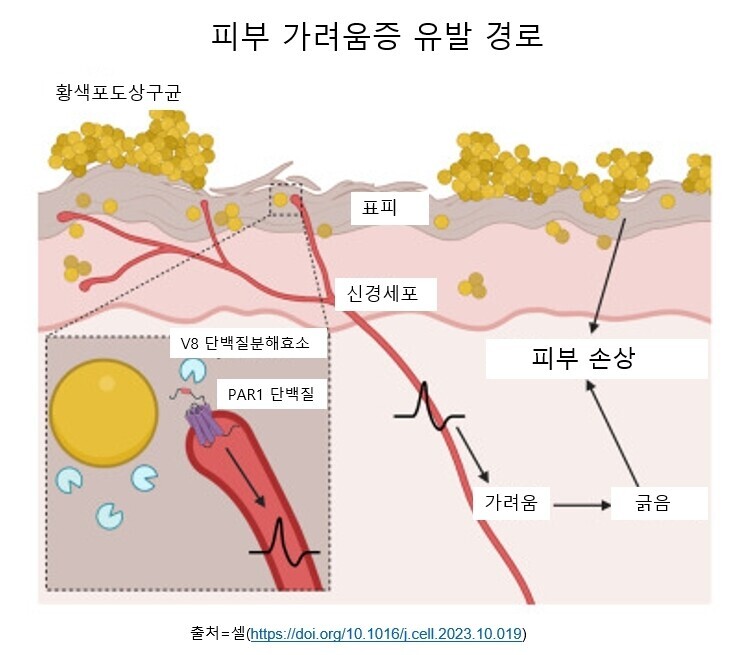
박테리아 효소가 잠자는 ‘가려움 단백질’ 깨워
연구진은 실험에서 우선 생쥐 피부에 황색포도상구균을 노출시켰다. 그러자 가려움증을 느낀 생쥐들은 피부를 긁기 시작했고 피부를 긁는 행동은 날이 갈수록 심해졌다. 그에 따라 피부 손상이 악화돼 가려움증을 느끼는 부위도 점차 넓어졌다. 나중엔 가려움증을 유발하지 않는 약한 자극에도 과민 반응을 보였다. 알로크네시스(alloknesis)라고 부르는 이런 과민 반응은 만성 피부 질환 환자에게서 흔히 나타나는 현상이다.연구진은 황색포도상구균이 가려움증을 유발하는 메카니즘을 확인하기 위해, 이 미생물이 피부에 닿으면 방출한다고 알려진 효소 단백질 10가지 각각에 대한 쥐의 반응을 지켜봤다.박테리아 유전자를 조작해 효소를 하나씩 제거하면서 확인한 결과, V8이라는 단백질 분해 효소가 가려움증을 유발한다는 사실이 드러났다. 연구진은 이어 아토피 피부염을 앓고 있는 사람 피부에서 다른 사람보다 황색포도상구균이 더 많으며, V8 수치도 더 높다는 걸 확인했다.분석 결과, V8 효소는 피부 신경세포의 PAR1이라는 단백질을 활성화해 가려움증을 유발하는 것으로 나타났다. 척수에서 유래한 이 단백질은 피부가 느끼는 촉감이나 열, 통증, 가려움증 같은 감각 신호를 뇌로 전달해주는 물질이다.이 단백질은 평소엔 휴면 상태이지만 V8 같은 특정 효소를 만나면 깨어난다. 연구진은 V8 효소가 이 단백질의 한 쪽 끝을 잘라내는 방식으로 단백질을 휴면에서 깨운다고 밝혔다. 생쥐 실험에서 이 단백질이 활성화하면 뇌가 가려움증으로 인식하는 신호가 시작되는 것으로 나타났다. 인간 신경세포를 이용한 실험에서도 신경세포는 V8 효소에 같은 반응을 보였다.
스테로이드 연고 안듣는 피부염 치료에 희소식
연구를 이끈 하버드의대의 아이작 추 교수(면역학)는 “이번 연구는 가려움증의 이면에 있는 완전히 새로운 메카니즘을 확인한 것”이라며 “그것은 바로 만성 아토피성 피부염을 앓고 있는 거의 모든 환자들한테서 발견되는 황색포도상구균”이라고 말했다.흥미로운 건 그동안 피부 알레르기나 가려움증을 유발하는 것으로 알려진 비만 세포, 호염기구 같은 면역 세포는 이번 실험에서 박테리아에 노출된 후에도 가려움증을 유발하지 않았다는 점이다.연구진은 황색포도상구균이 활성화하는 단백질 PAR1이 혈액 응고에도 관여한다는 점에 착안해 치료 방법도 찾아냈다. 황색포도상구균에 노출시킨 생쥐에게 항응고제를 바르자 증상이 빠르게 개선되는 걸 확인했다.연구진은 “현재 혈전 예방용으로 쓰이는 항응고제(PAR1 차단제)를 용도를 바꿔 가려움증 치료에도 쓸 수 있을 것”이라고 밝혔다. 연구진은 이번 연구가 아토피 피부염, 결절성 피부염, 건선 등 피부 미생물 군집의 불균형과 관련된 다양한 질환에서 발생하는 가려움증을 치료하기 위한 알약이나 연고제 개발에 유용한 정보가 될 것으로 기대했다.이번 연구는 그동안 베일에 싸여 있던 가려움증의 메카니즘을 확인했다는 점에서 큰 의미가 있다. 연구에 참여하지 않은 존스홉킨스의대 네이선 아처 교수(피부과학)는 ‘엔비시뉴스’에 “이번 연구는 염증 반응과 가려움증 반응을 분리해냈다”며 “스테로이드 연고 같은 치료법이 잘 듣지 않는 피부염 환자들을 치료하는 데 중요한 단서가 될 수 있다”고 말했다.그러나 이번 연구가 황색포도상구균이 가려움증의 유일한 원인균이라는 걸 말하는 것은 아니다.연구진은 “곰팡이, 바이러스, 박테리아를 포함한 많은 미생물이 가려움증을 동반한다”며 이들 미생물에 대해서도 어떻게 가려움·증을 유발하는지 연구할 계획이라고 밝혔다.이번 연구는 또 ‘미생물은 왜 가려움증을 유발할까’라는 좀 더 근본적인 질문을 제기한다. 진화론 측면에서 가려움증을 유발해 박테리아가 얻는 건 뭘까?연구진은 가려움증에 따른 숙주의 긁기 행동이 미생물의 확산 또는 번식에 유리하게 작용할 가능성이 있다고 말했다. 연구진은 결핵 박테리아가 미주신경을 자극해 기침을 유발함으로써 다른 숙주로 확산해가는 경우를 비슷한 사례로 들었다.“가려워서 긁는 행위는 왜 일어날까? 우리에게 도움이 될까, 아니면 미생물에게 도움이 될까?” 논문 제1저자인 리웬 덩 박사후연구원은 “앞으로 후속 연구를 통해 밝혀내야 할 부분”이라고 말했다.
Highlights
- •
S. aureus induces itch and scratch damage with epicutaneous skin exposure
- •
V8 protease (SspA) is necessary and sufficient for itch during S. aureus exposure
- •
S. aureus V8 activates mouse and human sensory neurons through PAR1
- •
PAR1 deficiency or blockade abrogates S. aureus-induced itch and skin damage
Summary
Graphical abstract
Keywords
Purchase one-time access:
Academic & Personal: 24 hour online accessCorporate R&D Professionals: 24 hour online accessRead-It-Now
Purchase access to all full-text HTML articles for 6 or 36 hr at a low cost. Click here to explore this opportunity.
One-time access price infoSubscribe:
Subscribe to CellReferences
-
- LaMotte R.H.
- Dong X.
- Ringkamp M.
Sensory neurons and circuits mediating itch.Nat. Rev. Neurosci. 2014; 15: 19-31https://doi.org/10.1038/nrn3641 -
- Bautista D.M.
- Wilson S.R.
- Hoon M.A.
Why we scratch an itch: the molecules, cells and circuits of itch.Nat. Neurosci. 2014; 17: 175-182https://doi.org/10.1038/nn.3619 -
- Wang F.
- Kim B.S.
Itch: a paradigm of neuroimmune crosstalk.Immunity. 2020; 52: 753-766https://doi.org/10.1016/j.immuni.2020.04.008 -
- Hwang J.
- Jaros J.
- Shi V.Y.
Staphylococcus aureus in atopic dermatitis: past, present, and future.Dermatitis. 2020; 31: 247-258https://doi.org/10.1097/DER.0000000000000589 -
- Campione E.
- Lanna C.
- Diluvio L.
- Cannizzaro M.V.
- Grelli S.
- Galluzzo M.
- Talamonti M.
- Annicchiarico-Petruzzelli M.
- Mancini M.
- Melino G.
- et al.
Skin immunity and its dysregulation in atopic dermatitis, hidradenitis suppurativa and vitiligo.Cell Cycle. 2020; 19: 257-267https://doi.org/10.1080/15384101.2019.1707455 -
- Geoghegan J.A.
- Irvine A.D.
- Foster T.J.
Staphylococcus aureus and atopic dermatitis: a complex and evolving relationship.Trends Microbiol. 2018; 26: 484-497https://doi.org/10.1016/j.tim.2017.11.008 -
- Blicharz L.
- Usarek P.
- Młynarczyk G.
- Skowroński K.
- Rudnicka L.
- Samochocki Z.
Is itch intensity in atopic dermatitis associated with skin colonization by Staphylococcus aureus?.Indian J. Dermatol. 2020; 65: 17-21https://doi.org/10.4103/ijd.IJD_136_19 -
- Johnson M.K.
Impetigo.Adv Emerg Nurs J. 2020; 42: 262-269 -
- Seilie E.S.
- Bubeck Wardenburg J.
Staphylococcus aureus pore-forming toxins: the interface of pathogen and host complexity.Semin. Cell Dev. Biol. 2017; 72: 101-116https://doi.org/10.1016/j.semcdb.2017.04.003 -
- Pietrocola G.
- Nobile G.
- Rindi S.
- Speziale P.
Staphylococcus aureus manipulates innate immunity through own and host-expressed proteases.Front. Cell. Infect. Microbiol. 2017; 7: 166https://doi.org/10.3389/fcimb.2017.00166 -
- Hatlen T.J.
- Miller L.G.
Staphylococcal skin and soft tissue infections.Infect. Dis. Clin. North Am. 2021; 35: 81-105https://doi.org/10.1016/j.idc.2020.10.003 -
- Chiu I.M.
- Heesters B.A.
- Ghasemlou N.
- Von Hehn C.A.
- Zhao F.
- Tran J.
- Wainger B.
- Strominger A.
- Muralidharan S.
- Horswill A.R.
- et al.
Bacteria activate sensory neurons that modulate pain and inflammation.Nature. 2013; 501: 52-57https://doi.org/10.1038/nature12479 -
- Blake K.J.
- Baral P.
- Voisin T.
- Lubkin A.
- Pinho-Ribeiro F.A.
- Adams K.L.
- Roberson D.P.
- Ma Y.C.
- Otto M.
- Woolf C.J.
- et al.
Staphylococcus aureus produces pain through pore-forming toxins and neuronal TRPV1 that is silenced by QX-314.Nat. Commun. 2018; 9: 37https://doi.org/10.1038/s41467-017-02448-6 -
- Pinho-Ribeiro F.A.
- Baddal B.
- Haarsma R.
- O'Seaghdha M.
- Yang N.J.
- Blake K.J.
- Portley M.
- Verri W.A.
- Dale J.B.
- Wessels M.R.
- et al.
Blocking neuronal signaling to immune cells treats streptococcal invasive infection.Cell. 2018; 173: 1083-1097.e22https://doi.org/10.1016/j.cell.2018.04.006 -
- Harrison I.P.
- Spada F.
Breaking the itch-scratch cycle: topical options for the management of chronic cutaneous itch in atopic dermatitis.Medicines (Basel). 2019; 6https://doi.org/10.3390/medicines6030076 -
- Kwatra S.G.
Breaking the itch-scratch cycle in prurigo nodularis.N. Engl. J. Med. 2020; 382: 757-758https://doi.org/10.1056/NEJMe1916733 -
- Elewski B.
- Alexis A.F.
- Lebwohl M.
- Stein Gold L.
- Pariser D.
- Del Rosso J.
- Yosipovitch G.
Itch: an under-recognized problem in psoriasis.J. Eur. Acad. Dermatol. Venereol. 2019; 33: 1465-1476https://doi.org/10.1111/jdv.15450 -
- Ross S.E.
Pain and itch: insights into the neural circuits of aversive somatosensation in health and disease.Curr. Opin. Neurobiol. 2011; 21: 880-887https://doi.org/10.1016/j.conb.2011.10.012 -
- Yosipovitch G.
- Greaves M.W.
- Schmelz M.
Itch.Lancet. 2003; 361: 690-694https://doi.org/10.1016/S0140-6736(03)12570-6 -
- Liu H.
- Archer N.K.
- Dillen C.A.
- Wang Y.
- Ashbaugh A.G.
- Ortines R.V.
- Kao T.
- Lee S.K.
- Cai S.S.
- Miller R.J.
- et al.
Staphylococcus aureus epicutaneous exposure drives skin inflammation via IL-36-mediated T cell responses.Cell Host Microbe. 2017; 22: 653-666.e5https://doi.org/10.1016/j.chom.2017.10.006 -
- Patrick G.J.
- Liu H.
- Alphonse M.P.
- Dikeman D.A.
- Youn C.
- Otterson J.C.
- Wang Y.
- Ravipati A.
- Mazhar M.
- Denny G.
- et al.
Epicutaneous Staphylococcus aureus induces IL-36 to enhance IgE production and ensuing allergic disease.J. Clin. Invest. 2021; 131https://doi.org/10.1172/JCI143334 -
- Nakatsuji T.
- Hata T.R.
- Tong Y.
- Cheng J.Y.
- Shafiq F.
- Butcher A.M.
- Salem S.S.
- Brinton S.L.
- Rudman Spergel A.K.
- Johnson K.
- et al.
Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial.Nat. Med. 2021; 27: 700-709https://doi.org/10.1038/s41591-021-01256-2 -
- Nakagawa S.
- Matsumoto M.
- Katayama Y.
- Oguma R.
- Wakabayashi S.
- Nygaard T.
- Saijo S.
- Inohara N.
- Otto M.
- Matsue H.
- et al.
Staphylococcus aureus virulent PSMα peptides induce keratinocyte alarmin release to orchestrate IL-17-dependent skin inflammation.Cell Host Microbe. 2017; 22: 667-677.e5https://doi.org/10.1016/j.chom.2017.10.008 -
- Moran G.J.
- Krishnadasan A.
- Gorwitz R.J.
- Fosheim G.E.
- McDougal L.K.
- Carey R.B.
- Talan D.A.
- EMERGEncy ID Net Study Group
Methicillin-resistant S. aureus infections among patients in the emergency department.N. Engl. J. Med. 2006; 355: 666-674https://doi.org/10.1056/NEJMoa055356 -
- Andersen H.H.
- Akiyama T.
- Nattkemper L.A.
- van Laarhoven A.
- Elberling J.
- Yosipovitch G.
- Arendt-Nielsen L.
Alloknesis and hyperknesis-mechanisms, assessment methodology, and clinical implications of itch sensitization.Pain. 2018; 159: 1185-1197https://doi.org/10.1097/j.pain.0000000000001220 -
- Ikoma A.
- Fartasch M.
- Heyer G.
- Miyachi Y.
- Handwerker H.
- Schmelz M.
Painful stimuli evoke itch in patients with chronic pruritus: central sensitization for itch.Neurology. 2004; 62: 212-217https://doi.org/10.1212/wnl.62.2.212 -
- Feng J.
- Luo J.
- Yang P.
- Du J.
- Kim B.S.
- Hu H.
Piezo2 channel-Merkel cell signaling modulates the conversion of touch to itch.Science. 2018; 360: 530-533https://doi.org/10.1126/science.aar5703 -
- Akiyama T.
- Carstens M.I.
- Ikoma A.
- Cevikbas F.
- Steinhoff M.
- Carstens E.
Mouse model of touch-evoked itch (alloknesis).J. Invest. Dermatol. 2012; 132: 1886-1891https://doi.org/10.1038/jid.2012.52 -
- Takanami K.
- Uta D.
- Matsuda K.I.
- Kawata M.
- Carstens E.
- Sakamoto T.
- Sakamoto H.
Estrogens influence female itch sensitivity via the spinal gastrin-releasing peptide receptor neurons.Proc. Natl. Acad. Sci. USA. 2021; 118https://doi.org/10.1073/pnas.2103536118 -
- Rimoin L.P.
- Kwatra S.G.
- Yosipovitch G.
Female-specific pruritus from childhood to postmenopause: clinical features, hormonal factors, and treatment considerations.Dermatol. Ther. 2013; 26: 157-167https://doi.org/10.1111/dth.12034 -
- Adams S.C.
- Garner J.P.
- Felt S.A.
- Geronimo J.T.
- Chu D.K.
A “Pedi” cures all: toenail trimming and the treatment of ulcerative dermatitis in mice.PLoS One. 2016; 11e0144871https://doi.org/10.1371/journal.pone.0144871 -
- Dong X.
- Dong X.
Peripheral and central mechanisms of itch.Neuron. 2018; 98: 482-494https://doi.org/10.1016/j.neuron.2018.03.023 -
- Oetjen L.K.
- Mack M.R.
- Feng J.
- Whelan T.M.
- Niu H.
- Guo C.J.
- Chen S.
- Trier A.M.
- Xu A.Z.
- Tripathi S.V.
- et al.
Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch.Cell. 2017; 171: 217-228.e13https://doi.org/10.1016/j.cell.2017.08.006 -
- Siracusa M.C.
- Kim B.S.
- Spergel J.M.
- Artis D.
Basophils and allergic inflammation.J. Allergy Clin. Immunol. 2013; 132 (; quiz 788): 789-801https://doi.org/10.1016/j.jaci.2013.07.046 -
- Obata K.
- Mukai K.
- Tsujimura Y.
- Ishiwata K.
- Kawano Y.
- Minegishi Y.
- Watanabe N.
- Karasuyama H.
Basophils are essential initiators of a novel type of chronic allergic inflammation.Blood. 2007; 110: 913-920https://doi.org/10.1182/blood-2007-01-068718 -
- Garcovich S.
- Maurelli M.
- Gisondi P.
- Peris K.
- Yosipovitch G.
- Girolomoni G.
Pruritus as a distinctive feature of type 2 inflammation.Vaccines (Basel). 2021; 9https://doi.org/10.3390/vaccines9030303 -
- Silva C.R.
- Melo B.M.S.
- Silva J.R.
- Lopes A.H.
- Pereira J.A.
- Cecilio N.T.
- Berlink J.
- Souza G.G.
- Lucas G.
- Vogl T.
- et al.
S100A9 plays a pivotal role in a mouse model of herpetic neuralgia via TLR4/TNF pathway.Brain Behav. Immun. 2020; 88: 353-362https://doi.org/10.1016/j.bbi.2020.03.033 -
- Shinkai Y.
- Rathbun G.
- Lam K.P.
- Oltz E.M.
- Stewart V.
- Mendelsohn M.
- Charron J.
- Datta M.
- Young F.
- Stall A.M.
RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement.Cell. 1992; 68: 855-867https://doi.org/10.1016/0092-8674(92)90029-c -
- Cao X.
- Shores E.W.
- Hu-Li J.
- Anver M.R.
- Kelsall B.L.
- Russell S.M.
- Drago J.
- Noguchi M.
- Grinberg A.
- Bloom E.T.
Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain.Immunity. 1995; 2: 223-238https://doi.org/10.1016/1074-7613(95)90047-0 -
- Garibyan L.
- Rheingold C.G.
- Lerner E.A.
Understanding the pathophysiology of itch.Dermatol. Ther. 2013; 26: 84-91https://doi.org/10.1111/dth.12025 -
- Akopian A.N.
- Sivilotti L.
- Wood J.N.
A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons.Nature. 1996; 379: 257-262https://doi.org/10.1038/379257a0 -
- Sangameswaran L.
- Delgado S.G.
- Fish L.M.
- Koch B.D.
- Jakeman L.B.
- Stewart G.R.
- Sze P.
- Hunter J.C.
- Eglen R.M.
- Herman R.C.
Structure and function of a novel voltage-gated, tetrodotoxin-resistant sodium channel specific to sensory neurons.J. Biol. Chem. 1996; 271: 5953-5956https://doi.org/10.1074/jbc.271.11.5953 -
- Jenul C.
- Horswill A.R.
Regulation of Staphylococcus aureus virulence.Microbiol. Spectr. 2019; 7https://doi.org/10.1128/microbiolspec.GPP3-0031-2018 -
- Mack M.R.
- Kim B.S.
The itch-scratch cycle: a neuroimmune perspective.Trends Immunol. 2018; 39: 980-991https://doi.org/10.1016/j.it.2018.10.001 -
- Akiyama T.
- Lerner E.A.
- Carstens E.
Protease-activated receptors and itch.Handb. Exp. Pharmacol. 2015; 226: 219-235https://doi.org/10.1007/978-3-662-44605-8_13 -
- Gimza B.D.
- Jackson J.K.
- Frey A.M.
- Budny B.G.
- Chaput D.
- Rizzo D.N.
- Shaw L.N.
Unraveling the impact of secreted proteases on hypervirulence in Staphylococcus aureus.mBio. 2021; 12https://doi.org/10.1128/mBio.03288-20 -
- Wörmann M.E.
- Reichmann N.T.
- Malone C.L.
- Horswill A.R.
- Gründling A.
Proteolytic cleavage inactivates the Staphylococcus aureus lipoteichoic acid synthase.J. Bacteriol. 2011; 193: 5279-5291https://doi.org/10.1128/JB.00369-11 -
- Williams M.R.
- Costa S.K.
- Zaramela L.S.
- Khalil S.
- Todd D.A.
- Winter H.L.
- Sanford J.A.
- O'Neill A.M.
- Liggins M.C.
- Nakatsuji T.
- et al.
Quorum sensing between bacterial species on the skin protects against epidermal injury in atopic dermatitis.Sci. Transl. Med. 2019; 11https://doi.org/10.1126/scitranslmed.aat8329 -
- Rice K.
- Peralta R.
- Bast D.
- de Azavedo J.
- McGavin M.J.
Description of staphylococcus serine protease (ssp) operon in Staphylococcus aureus and nonpolar inactivation of sspA-encoded serine protease.Infect. Immun. 2001; 69: 159-169https://doi.org/10.1128/IAI.69.1.159-169.2001 -
- Shimada S.G.
- LaMotte R.H.
Behavioral differentiation between itch and pain in mouse.Pain. 2008; 139: 681-687https://doi.org/10.1016/j.pain.2008.08.002 -
- Yamanoi Y.
- Kittaka H.
- Tominaga M.
Cheek injection model for simultaneous measurement of pain and itch-related behaviors.J. Vis. Exp. 2019;https://doi.org/10.3791/58943 -
- Chandrabalan A.
- Ramachandran R.
Molecular mechanisms regulating Proteinase-Activated Receptors (PARs).FEBS Journal. 2021; 288: 2697-2726https://doi.org/10.1111/febs.15829 -
- Coughlin S.R.
Thrombin signalling and protease-activated receptors.Nature. 2000; 407: 258-264https://doi.org/10.1038/35025229 -
- Coughlin S.R.
Protease-activated receptors in hemostasis, thrombosis and vascular biology.J. Thromb. Haemost. 2005; 3: 1800-1814https://doi.org/10.1111/j.1538-7836.2005.01377.x -
- Nakanishi-Matsui M.
- Zheng Y.W.
- Sulciner D.J.
- Weiss E.J.
- Ludeman M.J.
- Coughlin S.R.
PAR3 is a cofactor for PAR4 activation by thrombin.Nature. 2000; 404: 609-613https://doi.org/10.1038/35007085 -
- Chandrabalan A.
- Firth A.
- Litchfield R.B.
- Appleton C.T.
- Getgood A.
- Ramachandran R.
Identification of Proteinase Activated Receptor (PAR) cleaving enzymes in human osteoarthritis knee joint synovial fluids.bioRxiv. 2020; (Preprint at)https://doi.org/10.1101/2020.10.21.336693 -
- Markert Y.
- Köditz J.
- Ulbrich-Hofmann R.
- Arnold U.
Proline versus charge concept for protein stabilization against proteolytic attack.Protein Eng. 2003; 16: 1041-1046https://doi.org/10.1093/protein/gzg136 -
- Martin L.
- Augé C.
- Boué J.
- Buresi M.C.
- Chapman K.
- Asfaha S.
- Andrade-Gordon P.
- Steinhoff M.
- Cenac N.
- Dietrich G.
- et al.
Thrombin receptor: an endogenous inhibitor of inflammatory pain, activating opioid pathways.Pain. 2009; 146: 121-129https://doi.org/10.1016/j.pain.2009.07.016 -
- Desormeaux C.
- Bautzova T.
- Garcia-Caraballo S.
- Rolland C.
- Barbaro M.R.
- Brierley S.M.
- Barbara G.
- Vergnolle N.
- Cenac N.
Protease-activated receptor 1 is implicated in irritable bowel syndrome mediators-induced signaling to thoracic human sensory neurons.Pain. 2018; 159: 1257-1267https://doi.org/10.1097/j.pain.0000000000001208 -
- Bahou W.F.
- Nierman W.C.
- Durkin A.S.
- Potter C.L.
- Demetrick D.J.
Chromosomal assignment of the human thrombin receptor gene: localization to region q13 of chromosome 5.Blood. 1993; 82: 1532-1537 -
- Zeisel A.
- Hochgerner H.
- Lönnerberg P.
- Johnsson A.
- Memic F.
- van der Zwan J.
- Häring M.
- Braun E.
- Borm L.E.
- La Manno G.
- et al.
Molecular architecture of the mouse nervous system.Cell. 2018; 174: 999-1014.e22https://doi.org/10.1016/j.cell.2018.06.021 -
- Hill R.Z.
- Morita T.
- Brem R.B.
- Bautista D.M.
S1PR3 mediates itch and pain via distinct TRP channel-dependent pathways.J. Neurosci. 2018; 38: 7833-7843https://doi.org/10.1523/JNEUROSCI.1266-18.2018 -
- Akiyama T.
- Carstens E.
Neural processing of itch.Neuroscience. 2013; 250: 697-714https://doi.org/10.1016/j.neuroscience.2013.07.035 -
- Goswami S.C.
- Thierry-Mieg D.
- Thierry-Mieg J.
- Mishra S.
- Hoon M.A.
- Mannes A.J.
- Iadarola M.J.
Itch-associated peptides: RNA-Seq and bioinformatic analysis of natriuretic precursor peptide B and gastrin releasing peptide in dorsal root and trigeminal ganglia, and the spinal cord.Mol. Pain. 2014; 10: 44https://doi.org/10.1186/1744-8069-10-44 -
- Tavares-Ferreira D.
- Shiers S.
- Ray P.R.
- Wangzhou A.
- Jeevakumar V.
- Sankaranarayanan I.
- Cervantes A.M.
- Reese J.C.
- Chamessian A.
- Copits B.A.
- et al.
Spatial transcriptomics of dorsal root ganglia identifies molecular signatures of human nociceptors.Sci. Transl. Med. 2022; 14: eabj8186https://doi.org/10.1126/scitranslmed.abj8186 -
- Kunitz M.
Isolation of a crystalline protein compound of trypsin and of soybean trypsin-inhibitor.J. Gen. Physiol. 1947; 30: 311-320https://doi.org/10.1085/jgp.30.4.311 -
- Frydrych I.
- Mlejnek P.
Serine protease inhibitors N-alpha-tosyl-L-lysinyl-chloromethylketone (TLCK) and N-tosyl-L-phenylalaninyl-chloromethylketone (TPCK) are potent inhibitors of activated caspase proteases.J. Cell. Biochem. 2008; 103: 1646-1656https://doi.org/10.1002/jcb.21550 -
- Chackalamannil S.
- Wang Y.
- Greenlee W.J.
- Hu Z.
- Xia Y.
- Ahn H.S.
- Boykow G.
- Hsieh Y.
- Palamanda J.
- Agans-Fantuzzi J.
- et al.
Discovery of a novel, orally active himbacine-based thrombin receptor antagonist (SCH 530348) with potent antiplatelet activity.J. Med. Chem. 2008; 51: 3061-3064https://doi.org/10.1021/jm800180e -
- Liu Q.
- Dong X.
The role of the Mrgpr receptor family in itch.Handb. Exp. Pharmacol. 2015; 226: 71-88https://doi.org/10.1007/978-3-662-44605-8_5 -
- Zhao J.
- Munanairi A.
- Liu X.Y.
- Zhang J.
- Hu L.
- Hu M.
- Bu D.
- Liu L.
- Xie Z.
- Kim B.S.
- et al.
PAR2 mediates itch via TRPV3 signaling in keratinocytes.J. Invest. Dermatol. 2020; 140: 1524-1532https://doi.org/10.1016/j.jid.2020.01.012 -
- Warwick C.
- Cassidy C.
- Hachisuka J.
- Wright M.C.
- Baumbauer K.M.
- Adelman P.C.
- Lee K.H.
- Smith K.M.
- Sheahan T.D.
- Ross S.E.
- et al.
MrgprdCre lineage neurons mediate optogenetic allodynia through an emergent polysynaptic circuit.Pain. 2021; 162: 2120-2131https://doi.org/10.1097/j.pain.0000000000002227 -
- Tantry U.S.
- Bliden K.P.
- Chaudhary R.
- Novakovic M.
- Rout A.
- Gurbel P.A.
Vorapaxar in the treatment of cardiovascular diseases.Future Cardiol. 2020; 16: 373-384https://doi.org/10.2217/fca-2019-0090 -
- Gupta N.
- Liu R.
- Shin S.
- Sinha R.
- Pogliano J.
- Pogliano K.
- Griffin J.H.
- Nizet V.
- Corriden R.
SCH79797 improves outcomes in experimental bacterial pneumonia by boosting neutrophil killing and direct antibiotic activity.J. Antimicrob. Chemother. 2018; 73: 1586-1594https://doi.org/10.1093/jac/dky033 -
- Martin 2nd, J.K.
- Sheehan J.P.
- Bratton B.P.
- Moore G.M.
- Mateus A.
- Li S.H.
- Kim H.
- Rabinowitz J.D.
- Typas A.
- Savitski M.M.
- et al.
A dual-mechanism antibiotic kills Gram-negative bacteria and avoids drug resistance.Cell. 2020; 181: 1518-1532.e14https://doi.org/10.1016/j.cell.2020.05.005 -
- Prasad L.
- Leduc Y.
- Hayakawa K.
- Delbaere L.T.
The structure of a universally employed enzyme: V8 protease from Staphylococcus aureus.Acta Crystallogr. D Biol. Crystallogr. 2004; 60: 256-259https://doi.org/10.1107/S090744490302599X -
- Wang B.
- McHugh B.J.
- Qureshi A.
- Campopiano D.J.
- Clarke D.J.
- Fitzgerald J.R.
- Dorin J.R.
- Weller R.
- Davidson D.J.
IL-1β-induced protection of keratinocytes against Staphylococcus aureus-secreted proteases is mediated by human β-defensin 2.J. Invest. Dermatol. 2017; 137: 95-105https://doi.org/10.1016/j.jid.2016.08.025 -
- Iida H.
- Takai T.
- Hirasawa Y.
- Kamijo S.
- Shimura S.
- Ochi H.
- Nishioka I.
- Maruyama N.
- Ogawa H.
- Okumura K.
- et al.
Epicutaneous administration of papain induces IgE and IgG responses in a cysteine protease activity-dependent manner.Allergol. Int. 2014; 63: 219-226https://doi.org/10.2332/allergolint.13-OA-0621 -
- Poh S.E.
- Koh W.L.C.
- Lim S.Y.D.
- Wang E.C.E.
- Yew Y.W.
- Common J.E.A.
- Oon H.H.
- Li H.
Expression of Staphylococcus aureus virulence factors in atopic dermatitis.JID Innov. 2022; 2: 100130https://doi.org/10.1016/j.xjidi.2022.100130 -
- Kot B.
- Piechota M.
- Jakubczak A.
- Gryzińska M.
- Witeska M.
- Grużewska A.
- Baran K.
- Denkiewicz P.
The prevalence of virulence determinants in methicillin-resistant Staphylococcus aureus isolated from different infections in hospitalized patients in Poland.Sci. Rep. 2022; 12: 5477https://doi.org/10.1038/s41598-022-09517-x -
- Khan S.
- Marasa B.S.
- Sung K.
- Nawaz M.
Genotypic characterization of clinical isolates of Staphylococcus aureus from Pakistan.Pathogens. 2021; 10https://doi.org/10.3390/pathogens10080918 -
- Ziebandt A.K.
- Kusch H.
- Degner M.
- Jaglitz S.
- Sibbald M.J.
- Arends J.P.
- Chlebowicz M.A.
- Albrecht D.
- Pantucek R.
- Doskar J.
- et al.
Proteomics uncovers extreme heterogeneity in the Staphylococcus aureus exoproteome due to genomic plasticity and variant gene regulation.Proteomics. 2010; 10: 1634-1644https://doi.org/10.1002/pmic.200900313 -
- Meyer T.C.
- Michalik S.
- Holtfreter S.
- Weiss S.
- Friedrich N.
- Völzke H.
- Kocher T.
- Kohler C.
- Schmidt F.
- Bröker B.M.
- et al.
A comprehensive view on the human antibody repertoire against Staphylococcus aureus antigens in the general population.Front. Immunol. 2021; 12: 651619https://doi.org/10.3389/fimmu.2021.651619 -
- Radke E.E.
- Brown S.M.
- Pelzek A.J.
- Fulmer Y.
- Hernandez D.N.
- Torres V.J.
- Thomsen I.P.
- Chiang W.K.
- Miller A.O.
- Shopsin B.
- et al.
Hierarchy of human IgG recognition within the Staphylococcus aureus immunome.Sci. Rep. 2018; 8: 13296https://doi.org/10.1038/s41598-018-31424-3 -
- Nho Y.
- Lawson K.
- Banovic F.
- Han L.
Staphylococcus aureus phenol-soluble modulins induce itch sensation.J. Dermatol. Sci. 2022; 107: 48-51https://doi.org/10.1016/j.jdermsci.2022.07.002 -
- Bao C.
- Chen O.
- Sheng H.
- Zhang J.
- Luo Y.
- Hayes B.W.
- Liang H.
- Liedtke W.
- Ji R.R.
- Abraham S.N.
A mast cell-thermoregulatory neuron circuit axis regulates hypothermia in anaphylaxis.Sci. Immunol. 2023; 8: eadc9417https://doi.org/10.1126/sciimmunol.adc9417 -
- Stefansson K.
- Brattsand M.
- Roosterman D.
- Kempkes C.
- Bocheva G.
- Steinhoff M.
- Egelrud T.
Activation of proteinase-activated receptor-2 by human kallikrein-related peptidases.J. Invest. Dermatol. 2008; 128: 18-25https://doi.org/10.1038/sj.jid.5700965 -
- Frateschi S.
- Camerer E.
- Crisante G.
- Rieser S.
- Membrez M.
- Charles R.P.
- Beermann F.
- Stehle J.C.
- Breiden B.
- Sandhoff K.
- et al.
PAR2 absence completely rescues inflammation and ichthyosis caused by altered CAP1/Prss8 expression in mouse skin.Nat. Commun. 2011; 2: 161https://doi.org/10.1038/ncomms1162 -
- Steinhoff M.
- Neisius U.
- Ikoma A.
- Fartasch M.
- Heyer G.
- Skov P.S.
- Luger T.A.
- Schmelz M.
Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin.J. Neurosci. 2003; 23: 6176-6180https://doi.org/10.1523/JNEUROSCI.23-15-06176.2003 -
- Williams M.R.
- Nakatsuji T.
- Sanford J.A.
- Vrbanac A.F.
- Gallo R.L.
Staphylococcus aureus induces increased serine protease activity in keratinocytes.J. Invest. Dermatol. 2017; 137: 377-384https://doi.org/10.1016/j.jid.2016.10.008 -
- Chua W.
- Poh S.E.
- Li H.
Secretory proteases of the human skin microbiome.Infect. Immun. 2022; 90e0039721https://doi.org/10.1128/IAI.00397-21 -
- Cau L.
- Williams M.R.
- Butcher A.M.
- Nakatsuji T.
- Kavanaugh J.S.
- Cheng J.Y.
- Shafiq F.
- Higbee K.
- Hata T.R.
- Horswill A.R.
- et al.
Staphylococcus epidermidis protease EcpA can be a deleterious component of the skin microbiome in atopic dermatitis.J. Allergy Clin. Immunol. 2021; 147: 955-966.e16https://doi.org/10.1016/j.jaci.2020.06.024 -
- Lukomski S.
- Montgomery C.A.
- Rurangirwa J.
- Geske R.S.
- Barrish J.P.
- Adams G.J.
- Musser J.M.
Extracellular cysteine protease produced by Streptococcus pyogenes participates in the pathogenesis of invasive skin infection and dissemination in mice.Infect. Immun. 1999; 67: 1779-1788https://doi.org/10.1128/IAI.67.4.1779-1788.1999 -
- Baral P.
- Udit S.
- Chiu I.M.
Pain and immunity: implications for host defence.Nat. Rev. Immunol. 2019; 19: 433-447https://doi.org/10.1038/s41577-019-0147-2 -
- Sorkin L.S.
- Eddinger K.A.
- Woller S.A.
- Yaksh T.L.
Origins of antidromic activity in sensory afferent fibers and neurogenic inflammation.Semin. Immunopathol. 2018; 40: 237-247https://doi.org/10.1007/s00281-017-0669-2 -
- Chiu I.M.
- von Hehn C.A.
- Woolf C.J.
Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology.Nat. Neurosci. 2012; 15: 1063-1067https://doi.org/10.1038/nn.3144 -
- Souza-Moreira L.
- Campos-Salinas J.
- Caro M.
- Gonzalez-Rey E.
Neuropeptides as pleiotropic modulators of the immune response.Neuroendocrinology. 2011; 94: 89-100https://doi.org/10.1159/000328636 -
- de Garavilla L.
- Vergnolle N.
- Young S.H.
- Ennes H.
- Steinhoff M.
- Ossovskaya V.S.
- D'Andrea M.R.
- Mayer E.A.
- Wallace J.L.
- Hollenberg M.D.
- et al.
Agonists of proteinase-activated receptor 1 induce plasma extravasation by a neurogenic mechanism.Br. J. Pharmacol. 2001; 133: 975-987https://doi.org/10.1038/sj.bjp.0704152 -
- Ruhl C.R.
- Pasko B.L.
- Khan H.S.
- Kindt L.M.
- Stamm C.E.
- Franco L.H.
- Hsia C.C.
- Zhou M.
- Davis C.R.
- Qin T.
- et al.
Mycobacterium tuberculosis Sulfolipid-1 activates nociceptive neurons and induces cough.Cell. 2020; 181 (e211): 293-305.e11https://doi.org/10.1016/j.cell.2020.02.026 -
- Bennett C.F.
- Kordasiewicz H.B.
- Cleveland D.W.
Antisense drugs make sense for neurological diseases.Annu. Rev. Pharmacol. Toxicol. 2021; 61: 831-852https://doi.org/10.1146/annurev-pharmtox-010919-023738 -
- Halvorsen J.A.
- Dalgard F.
- Thoresen M.
- Bjertness E.
- Lien L.
Itch and pain in adolescents are associated with suicidal ideation: a population-based cross-sectional study.Acta Derm. Venereol. 2012; 92: 543-546https://doi.org/10.2340/00015555-1251 -
- Schneider G.
- Driesch G.
- Heuft G.
- Evers S.
- Luger T.A.
- Ständer S.
Psychosomatic cofactors and psychiatric comorbidity in patients with chronic itch.Clin. Exp. Dermatol. 2006; 31: 762-767https://doi.org/10.1111/j.1365-2230.2006.02211.x -
- Frey A.M.
- Chaput D.
- Shaw L.N.
Insight into the human pathodegradome of the V8 protease from Staphylococcus aureus.Cell Rep. 2021; 35: 108930https://doi.org/10.1016/j.celrep.2021.108930 -
- Motta J.P.
- Denadai-Souza A.
- Sagnat D.
- Guiraud L.
- Edir A.
- Bonnart C.
- Sebbag M.
- Rousset P.
- Lapeyre A.
- Seguy C.
- et al.
Active thrombin produced by the intestinal epithelium controls mucosal biofilms.Nat. Commun. 2019; 10: 3224https://doi.org/10.1038/s41467-019-11140-w -
- Maurer K.
- Reyes-Robles T.
- Alonzo 3rd, F.
- Durbin J.
- Torres V.J.
- Cadwell K.
Autophagy mediates tolerance to Staphylococcus aureus alpha-toxin.Cell Host Microbe. 2015; 17: 429-440https://doi.org/10.1016/j.chom.2015.03.001 -
- Joo H.S.
- Cheung G.Y.
- Otto M.
Antimicrobial activity of community-associated methicillin-resistant Staphylococcus aureus is caused by phenol-soluble modulin derivatives.J. Biol. Chem. 2011; 286: 8933-8940https://doi.org/10.1074/jbc.M111.221382 -
- Austin C.M.
- Garabaglu S.
- Krute C.N.
- Ridder M.J.
- Seawell N.A.
- Markiewicz M.A.
- Boyd J.M.
- Bose J.L.
Contribution of YjbIH to virulence factor expression and host colonization in Staphylococcus aureus.Infect. Immun. 2019; 87https://doi.org/10.1128/IAI.00155-19 -
- Boucher A.A.
- Rosenfeldt L.
- Mureb D.
- Shafer J.
- Sharma B.K.
- Lane A.
- Crowther R.R.
- McKell M.C.
- Whitt J.
- Alenghat T.
- et al.
Cell type-specific mechanisms coupling protease-activated receptor-1 to infectious colitis pathogenesis.J. Thromb. Haemost. 2020; 18: 91-103https://doi.org/10.1111/jth.14641 -
- Liu Q.
- Tang Z.
- Surdenikova L.
- Kim S.
- Patel K.N.
- Kim A.
- Ru F.
- Guan Y.
- Weng H.J.
- Geng Y.
- et al.
Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus.Cell. 2009; 139: 1353-1365https://doi.org/10.1016/j.cell.2009.11.034 -
- Nassar M.A.
- Stirling L.C.
- Forlani G.
- Baker M.D.
- Matthews E.A.
- Dickenson A.H.
- Wood J.N.
Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain.Proc. Natl. Acad. Sci. USA. 2004; 101: 12706-12711https://doi.org/10.1073/pnas.0404915101 -
- Luong T.T.
- Lee C.Y.
Improved single-copy integration vectors for Staphylococcus aureus.J. Microbiol. Methods. 2007; 70: 186-190https://doi.org/10.1016/j.mimet.2007.04.007 -
- Crosby H.A.
- Schlievert P.M.
- Merriman J.A.
- King J.M.
- Salgado-Pabón W.
- Horswill A.R.
The Staphylococcus aureus global regulator MgrA modulates clumping and virulence by controlling surface protein expression.PLoS Pathog. 2016; 12e1005604https://doi.org/10.1371/journal.ppat.1005604 -
- Madisen L.
- Zwingman T.A.
- Sunkin S.M.
- Oh S.W.
- Zariwala H.A.
- Gu H.
- Ng L.L.
- Palmiter R.D.
- Hawrylycz M.J.
- Jones A.R.
- et al.
A robust and high-throughput Cre reporting and characterization system for the whole mouse brain.Nat. Neurosci. 2010; 13: 133-140https://doi.org/10.1038/nn.2467 -
- Hou B.
- Reizis B.
- DeFranco A.L.
Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms.Immunity. 2008; 29: 272-282https://doi.org/10.1016/j.immuni.2008.05.016 -
- Schmidlin F.
- Amadesi S.
- Dabbagh K.
- Lewis D.E.
- Knott P.
- Bunnett N.W.
- Gater P.R.
- Geppetti P.
- Bertrand C.
- Stevens M.E.
Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway.J. Immunol. 2002; 169: 5315-5321https://doi.org/10.4049/jimmunol.169.9.5315 -
- Valtcheva M.V.
- Copits B.A.
- Davidson S.
- Sheahan T.D.
- Pullen M.Y.
- McCall J.G.
- Dikranian K.
- Gereau R.W.t.
Surgical extraction of human dorsal root ganglia from organ donors and preparation of primary sensory neuron cultures.Nat. Protoc. 2016; 11: 1877-1888https://doi.org/10.1038/nprot.2016.111 -
- Monk I.R.
- Shah I.M.
- Xu M.
- Tan M.W.
- Foster T.J.
Transforming the untransformable: application of direct transformation to manipulate genetically Staphylococcus aureus and Staphylococcus epidermidis.mBio. 2012; 3https://doi.org/10.1128/mBio.00277-11 -
- Mootz J.M.
- Malone C.L.
- Shaw L.N.
- Horswill A.R.
Staphopains modulate Staphylococcus aureus biofilm integrity.Infect. Immun. 2013; 81: 3227-3238https://doi.org/10.1128/IAI.00377-13 -
- Deng L.
- Schilcher K.
- Burcham L.R.
- Kwiecinski J.M.
- Johnson P.M.
- Head S.R.
- Heinrichs D.E.
- Horswill A.R.
- Doran K.S.
Identification of key determinants of Staphylococcus aureus vaginal colonization.mBio. 2019; 10https://doi.org/10.1128/mBio.02321-19 -
- VanDrisse C.M.
- Escalante-Semerena J.C.
New high-cloning-efficiency vectors for complementation studies and recombinant protein overproduction in Escherichia coli and Salmonella enterica.Plasmid. 2016; 86: 1-6https://doi.org/10.1016/j.plasmid.2016.05.001 -
- Nesvizhskii A.I.
- Keller A.
- Kolker E.
- Aebersold R.
A statistical model for identifying proteins by tandem mass spectrometry.Anal Chem. 2003; 75: 4646-4658https://doi.org/10.1021/ac0341261 -
- DuBreuil D.M.
- Chiang B.M.
- Zhu K.
- Lai X.
- Flynn P.
- Sapir Y.
- Wainger B.J.
A high-content platform for physiological profiling and unbiased classification of individual neurons.Cell Rep. Methods. 2021; 1https://doi.org/10.1016/j.crmeth.2021.100004 -
- Yousuf M.S.
- Samtleben S.
- Lamothe S.M.
- Friedman T.N.
- Catuneanu A.
- Thorburn K.
- Desai M.
- Tenorio G.
- Schenk G.J.
- Ballanyi K.
- et al.
Endoplasmic reticulum stress in the dorsal root ganglia regulates large-conductance potassium channels and contributes to pain in a model of multiple sclerosis.FASEB J. 2020; 34: 12577-12598https://doi.org/10.1096/fj.202001163R -
- Voisin T.
- Perner C.
- Messou M.A.
- Shiers S.
- Ualiyeva S.
- Kanaoka Y.
- Price T.J.
- Sokol C.L.
- Bankova L.G.
- Austen K.F.
- et al.
The CysLT(2)R receptor mediates leukotriene C4-driven acute and chronic itch.Proc. Natl. Acad. Sci. USA. 2021; 118https://doi.org/10.1073/pnas.2022087118 -
- Mihara K.
- Ramachandran R.
- Renaux B.
- Saifeddine M.
- Hollenberg M.D.
Neutrophil elastase and proteinase-3 trigger G protein-biased signaling through proteinase-activated receptor-1 (PAR1).J. Biol. Chem. 2013; 288: 32979-32990https://doi.org/10.1074/jbc.M113.483123
